By Prof. Kai Vilanova. Chief MRI Department. Institute of Biomedical Research of Girona (IDIBGI)
Clinical Challenge: Can artificial intelligence based mpMRI with clinical data predict prostate cancer recurrence after prostatectomy?
Radical prostatectomy (RP) is a first-line treatment for organ-confined or locally advanced prostate cancer (PCa). Biochemical recurrence after surgery is defined as two consecutive PSA values ≥0.2 ng/ml after radical prostatectomy. Post-RP biochemical recurrence (BCR), affecting 50% of the patients, is a strong surrogate marker for indicating subsequent distant metastasis. BCR predominantly develops in patients with high-risk features, such as high initial prostate-specific antigen (PSA) levels, adverse RP pathology, including extracapsular extension (ECE) and seminal vesicle invasion (SVI), positive surgical margins (PSM), and surgical Gleason score (GS) ≥8.
Early identification of patients with PCa at a higher risk for developing post-RP BCR is important for intensive monitoring or providing adjuvant therapies at the proper time for effectively managing systemic micro-metastasis and ultimately securing long-term survival advantages mpMRI could be helpful to better stratify the disease-risk and support the physician in counseling the patient regarding treatment. ΜpMRI combined with advanced post-processing techniques seems to be an effective non-invasive method to identify the set of features characterizing increased risk for symptomatic and probably fatal prostate cancer.
Use Case (UC) 5 of the ProCAncer-I project, aims to predict the risk of disease recurrence after prostatectomy. Validated models are going to be used to evaluate the risk of disease recurrence, using pre and post-treatment mpMRI imaging studies both in vendor-specific and vendor-neutral models. In this task we are focusing on post-surgery findings as well as positive surgical margins. In addition, a nomogram comprising of a radiomic score and clinical variables that are known to be good predictors of disease recurrence (Gleason Score, status of resection margins, extracapsular extension) will be externally validated. Data is collected from patients who have undergone prostatectomy. The Case Report Form (eCRF) from UC 5 has three information sections: Clinical, Lesions, and Follow-up. In the section “Follow-up” (Figure: arrow) the user should provide the following information: Biochemical Relapse: true (checked) or false (not checked).
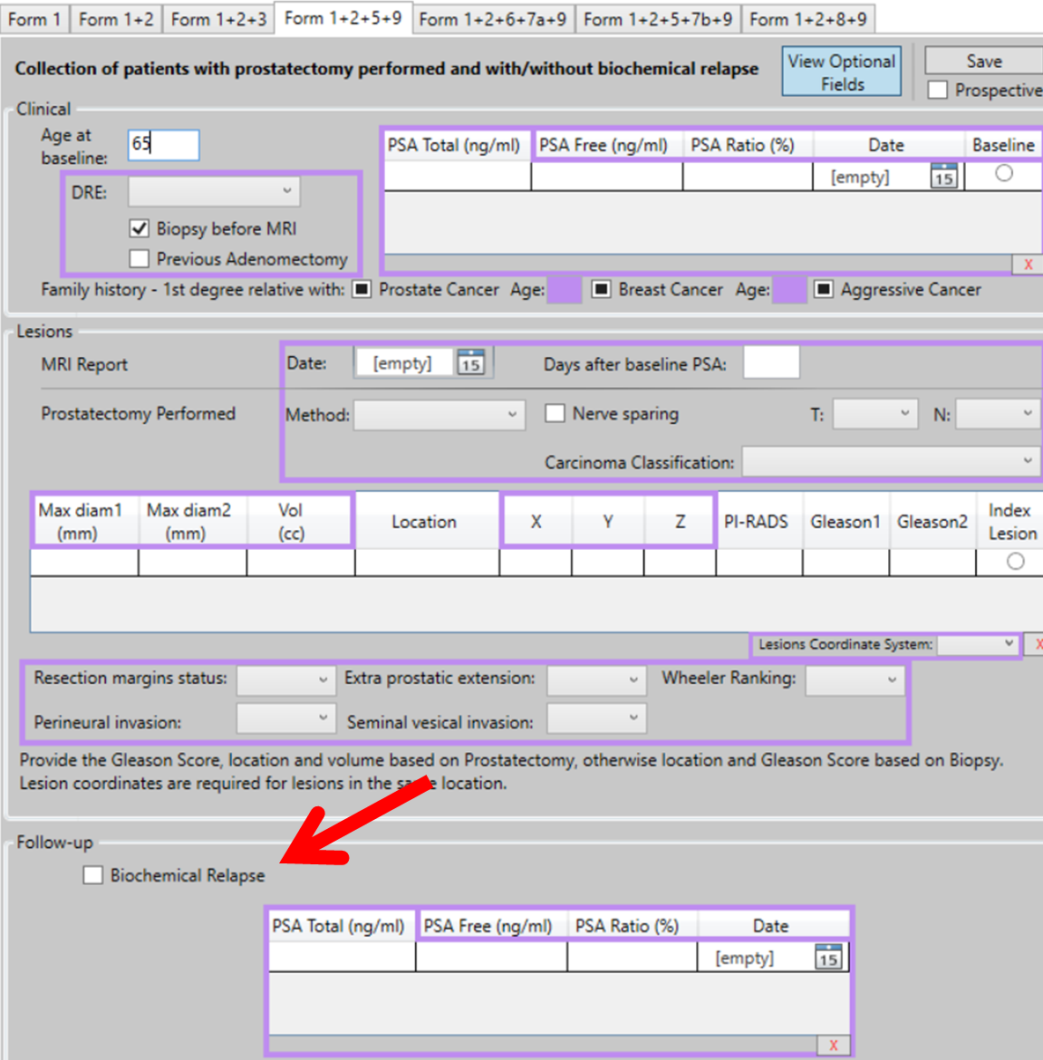
Figure: eCRF form for UC 5 collects clinical, MRI data and the PSA follow-up to consider biochemical recurrence/relapse

